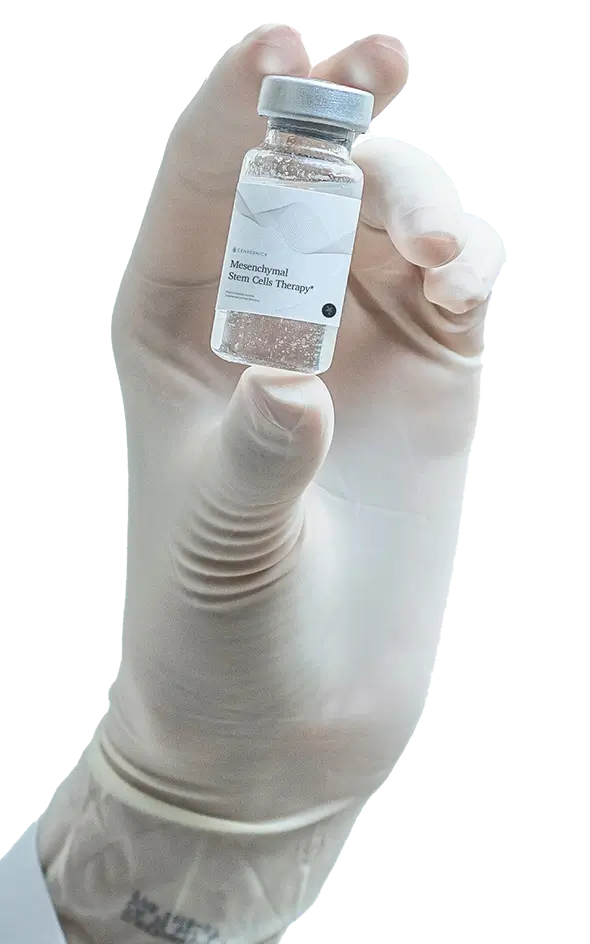

Our main regenerative therapy harnesses the properties of mesenchymal stem cells.

Regenerative therapy to address conditions such as chronic pain, osteoarthritis and musculoskeletal injuries.

State-of-the-art skin and hair rejuvenation for a more youthful appearance.


To create an optimal internal restorative environment, it can serve as an option or complement to drugs and surgical interventions, as well as a preventive and esthetic measure, solving each individual need*.
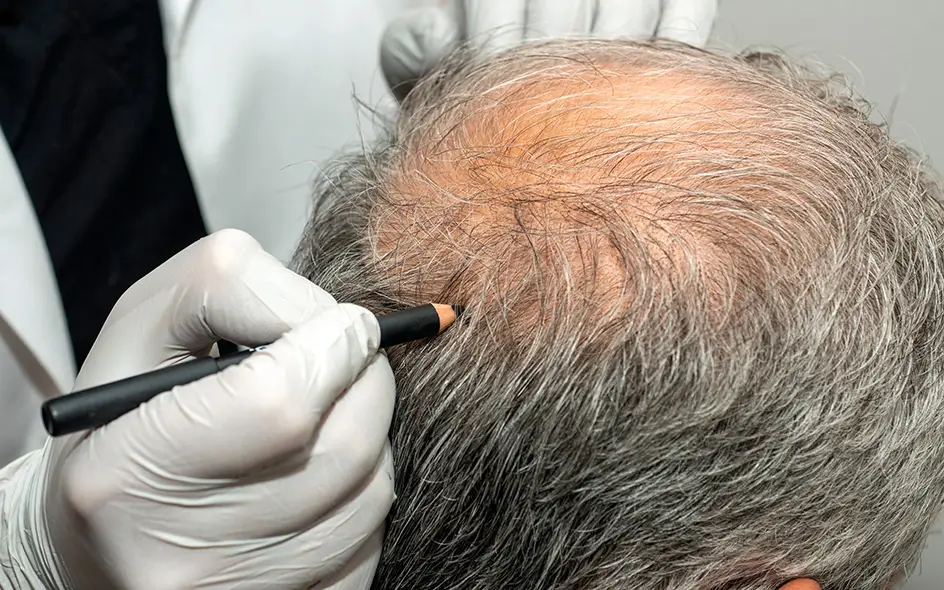
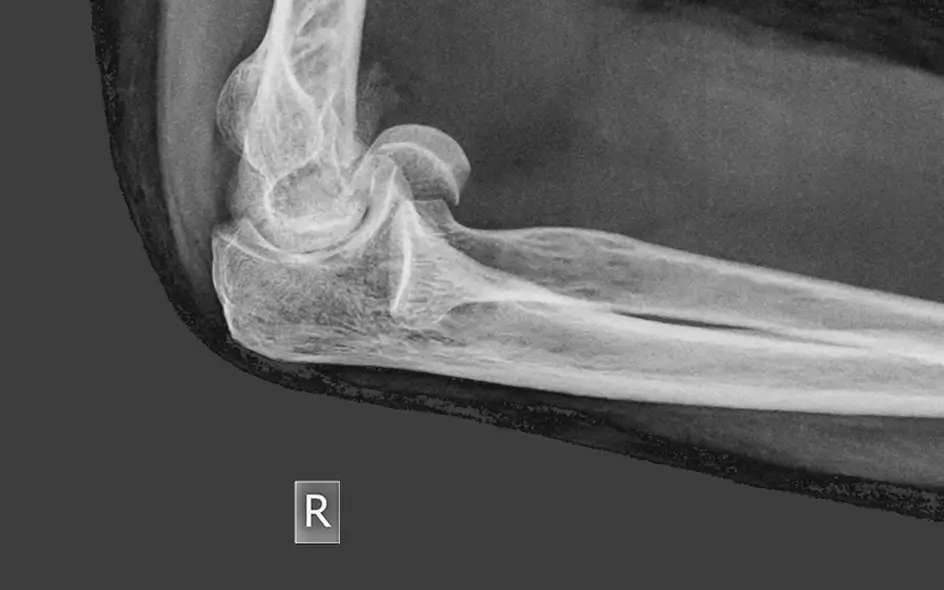

Many diseases are related to an imbalance in the inflammatory or immune status. A regenerative therapy could significantly benefit patients [7]. UC-MSCs have demonstrated clinical efficacy in the following diseases:
To participate in the therapy admission process, you will need to fill out an online eligibility form. This form will provide our medical team with the preliminary information needed to determine if you are eligible for our therapies.
The process consists of three steps that each applicant must follow before final approval:
Please fill out the eligibility form in the language of your choice. If you meet the requirements, we will begin the admission process.
